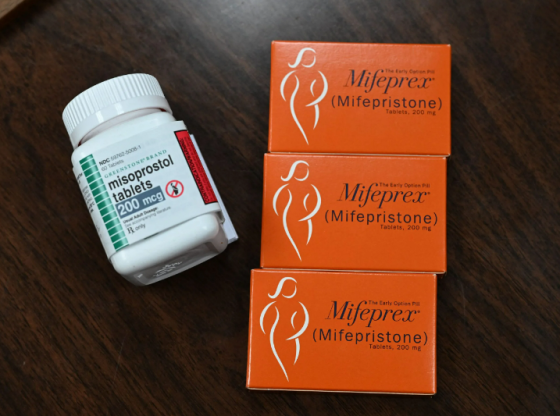
The Food and Drug Administration has made a regulatory change this week that will now make it possible for retail pharmacies to offer abortion pills. Previously, abortion pills were prescribed online and mailed to patients, to circumvent state restrictions. A judge, as early as next month, was on course to decide whether to freeze the FDA’s 2000 approval of mifepristone. But they FDA has moved forward by themselves and to loosend restrictions around mifepristone, which is taken alongside another drug misoprostol during the first 10 weeks of pregnancy.
The FDA has officially removed mifepristone’s in-person dispensing requirement and is allowing it to be sold directly from pharmacies. However, pharmacies might still need to comply with the laws of the state they are located in, some of which could restrict the ability to provide such pills.
The battle over mifepristone has many fronts though, as the group Alliance Defending Freedom filed a lawsuit in November, arguing that the FDA did not properly approve mifepristone for terminating pregnancies. The lawsuit states that the FDA didn’t conduct safety studies and deemed pregnancy an “illness” in order to gain approval for mifepristone.
If successful, the lawsuit would “would effectively result in a nationwide ban on this safe, effective, common method of abortion, even in those states that have protected access,” according to Carrie Flaxman, senior director at Planned Parenthood Federation of America.